Study Design
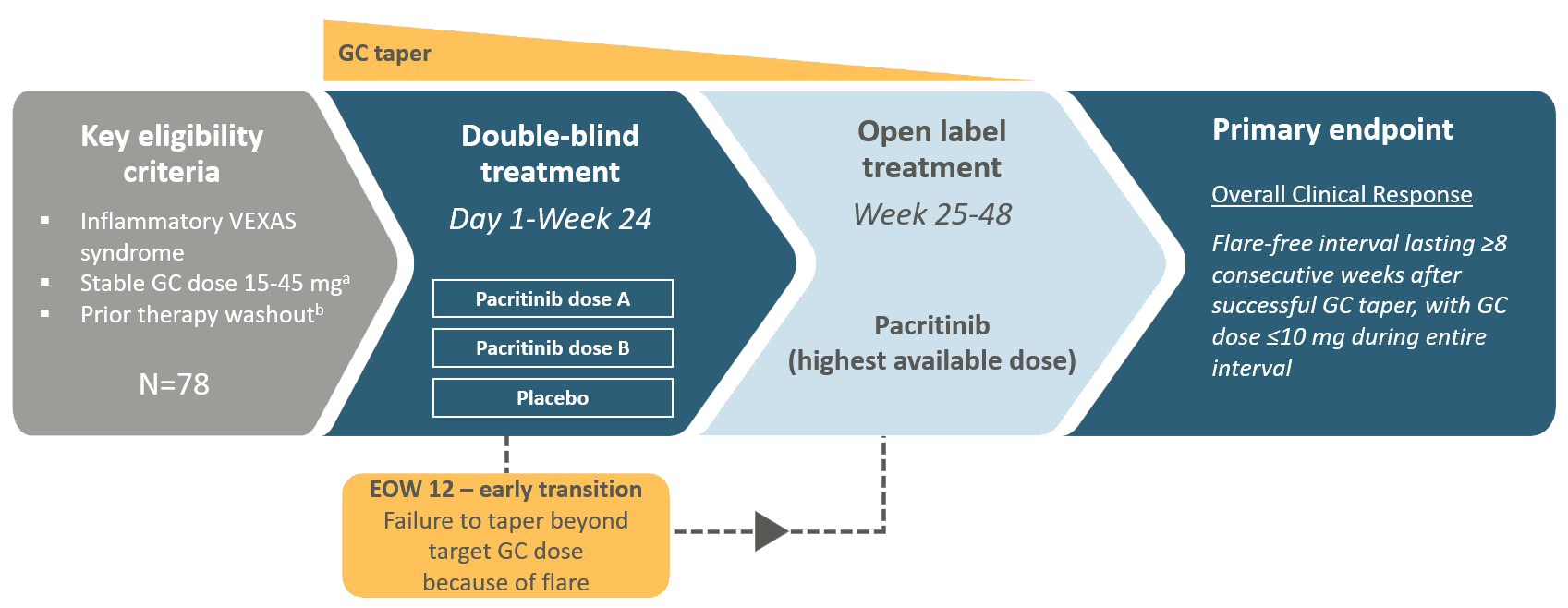
VEXAS, Vacuoles, E1 enzyme, X-linked, Autoinflammatory, Somatic; mg, milligrams; GC, Glucocorticoid; EOW, End of Week
aPatients who are stable on GC doses of 10-14 mg daily in addition to another non-GC anti-inflammatory therapy at Screening who have a previously documented VEXAS flare on GC monotherapy at a dose of ≥10 mg may be eligible, provided their GC dose is escalated to 15-45mg daily after washout.
bTime periods for washout are variable based on type of non-GC anti-inflammatory therapy